Leaf spot of Hosta ventricosa caused by Fusarium oxysporum in China
- Published
- Accepted
- Received
- Academic Editor
- Rana Muhammad Atif
- Subject Areas
- Molecular Biology, Mycology, Plant Science
- Keywords
- Fungi, Hosta ventricosa, Fusarium oxysporum, Multi gene phylogeny, New disease
- Copyright
- © 2021 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Leaf spot of Hosta ventricosa caused by Fusarium oxysporum in China. PeerJ 9:e12581 https://doi.org/10.7717/peerj.12581
Abstract
Leaf spot of Hosta ventricosa is a new disease in China. This disease seriously affects the ornamental value and greening function of H. ventricosa. Identification of the causal agent can prevent and control leaf spot in H. ventricosa and promote the healthy development of the H. ventricosa industry. Known incidents of leaf spot of H. ventricosa occurred in three places, and samples were collected. After the fungus were isolated, its pathogenicity was tested according to Koch’s postulates. Isolates ZE-1b and ZE-2b were identified as Fusarium oxysporum based on morphological features and multigene phylogenetic analyses of calmodulin (CMDA), RNA polymerase II subunit A (RPB1), RNA polymerase II second largest subunit (RPB2) and translation elongation factor 1-alpha (TEF1). These results provide a theoretical basis for the control of this disease of H. ventricosa.
Introduction
Hosta ventricosa, is a perennial herbaceous plant of the Hosta genus in Liliaceae. It originated in China, South Korea and Japan (Yu et al., 2015) and is widely distributed in China, including Jiangsu, Anhui, Hebei and other places (Liu et al., 2008). In addition to its bright leaves and graceful habit, this species has strong adaptability to the environment and is suitable for planting under trees, in the shade of buildings or other exposed shaded places. It is an excellent ground cover with ornamental value and greening functions (Zhao, Chen & Lv, 2009). In addition, the whole plant, flowers, leaves or roots can be used as a traditional Chinese medicinal material with the ability to dissipate blood stasis and relieve pain (Zeng, Zhao & Li, 2020).
However, H. ventricosa is impacted by several major pathogens, such as Sclerotium rolfsii Sacc. This disease manifests because the H. ventricosa leaves are especially thick, and during the rainy season, the H. ventricosa rhizome is in contact with water for a prolonged time (Zhao, Chen & Lv, 2009). Thus, the epidermis of the affected area becomes brown and necrotic, and finally a white mycelial layer is formed, which leads to cortex decay (Li et al., 2013). In addition, excessive humidity and poor drainage in the rainy season can also favor diseases caused by Colletotrichum, which mainly damages the leaves, petioles and pedicels of H. ventricosa. The plants present round or nearly round discrolloid spots that are gray or grayish brown (Zhao, Chen & Lv, 2009). Leaf spot of H. ventricosa caused by F. oxysporum is a very serious fungal disease (Fisher et al., 2012). F. oxysporum is one of the top ten most important plant pathogenic fungi in the world, with high virulence and a wide distribution area. The pathogen can cause plant drying and wilting. F. oxysporum can be a saprophytic or parasitic fungus. It is widely found in nature, animals and plants, and has been isolated in cold Arctic areas and arid deserts. This strain can cause wilt and decay of roots, stems, leaves, flowers and fruits in over one hundred plant hosts (Maryani et al., 2019).
Materials and Methods
Experimental materials
Leaves of infected H. ventricosa were collected in Nanjing from 2020 to 2021. Materials used in this study included PDA plates, tissue separation tools, 2% CTAB, and chloroform.
Sampling and isolation
To isolate the fungus, H. ventricose leaves showing leaf spots were collected from three places in Nanjing, China (32°4′47″N, 118°48′31″E; 32°4′45″N, 118°48′31″E; 32°4′44″N, 118°48′31″E.), in September 2020. The collected leaves were rinsed under tap water for 15–30 min. After the leaves were dried, both healthy and affected tissues were cut into small pieces, each of which was 2 × 2 mm in size. The pieces were disinfected in 75% ethanol for 30 s and in 2% NaClO for 90 s, then rinsed with sterile water 3 times for 20 s each time (Si et al., 2021), dried on sterile filter paper and inoculated onto PDA. After the appearance of fungal colonies, blocks of tissue were removed from the edges of the colonies for purification. The morphological characteristics, color, size and shape of the purified colonies were observed and described (Chang et al., 2020). Two single-spore cultures were used for further study and were also deposited in the China Forestry Culture Collection Center (CFCC).
Pathogenicity test
The experiments were replicated 3 times, and a total of 30 seedings were used. Healthy H. ventricosa leaves were collected and rinsed with clean water. The leaves surface were disinfected and dried on an aseptic bench. Pathogenic isolates were inoculated on PDA plates and cultured in an incubator at 25 °C for 5 days. To test the pathogenicity of the isolates, H. ventricosa leaves were wounded with a sterile needle and then inoculated with 5 mm plugs cut out from the growing edges of 5-day-old cultures (Feng et al., 2019). Three replicates were used (Yang et al., 2021b). At the same time, isolates were inoculated onto plants in the natural environment in the wild. Leaves mock inoculated without isolates were used as controls, and the incidence of leaf spot was observed after 3 days (Yang et al., 2021a).
Morphological analysis
Pathogenic isolates were inoculated on PDA plates and incubated in an incubator at 25 °C for 1 week to observe and record the morphology, color, surface characteristics and growth status at the edges of the colonies (Zhang, 2014). The morphology, size and presence of spore septations were recorded under a microscope (Murugan et al., 2020).
DNA extraction, amplification, sequencing and phylogenetic analyses
Before DNA extraction, a small portion of mycelia taken from a 7-day-old cultures of the pathogen grown on PDA plates at 25 °C was collected and transferred to 2-ml Eppendorf tubes. Genomic DNA was extracted by the CTAB method (Freeman, Katan & Shabi, 1996). After passing the test, the mycelia were stored at −18 °C (Guo, Hyde & Liew, 2000; Saghai-Maroof et al., 1984).
The extracted DNA was subjected to polymerase chain reaction (PCR) amplification of partial regions of four genes/regions, namely, calmodulin (CMDA), RNA polymerase II subunit A (RPB1), RNA polymerase II second largest subunit (RPB2) and translation elongation factor 1-alpha (TEF1), which were amplified with primers CL1/CL2A, FA/G2R, 5F2/7CR, and EF1/EF2, respectively (Table 1).
| Locus | Primer | PCR amplification procedures | Reference | |
|---|---|---|---|---|
| Designation sequence (5′ – 3′)* | ||||
| TEF1 | EF1 | ATGGGTAAGGARGACAAGAC | 94 °C to 90 s; Cycles of 94 °C 45 s, 55 °C 45 s, 72 °C 1 min; 72 °C for 10 min; Soak 10 °C | O’Donnell et al. (1998) |
| EF2 | GGARGTACCAGTSATCATG | O’Donnell et al. (1998) | ||
| CAMD | CL1 | GARTWCAAGGAGGCCTTCTC | 94 °C to 90 s; Cycles of 94 °C 45 s, 55 °C 45 s, 72 °C 1 min; 72 °C for 10 min; Soak 10 °C | Lombard et al. (2019) |
| CL2A | TTTTTGCATCATGAGTTGGAC | Lombard et al. (2019) | ||
| RPB1 | Fa | CAYAARGARTCYATGATGGGWC | 94 °C to 90 s; Cycles of 94 °C 45 s, 58 °C 45 s, 72 °C 2 min; Cycles of 94 °C 45 s, 57 °C 45 s, 72 °C 2 min; Cycles of 94 °C 45s, 56 °C 45 s, 72 °C 2 min; 72 °C for 10 min; Soak 10 °C | O’Donnell et al. (2010) |
| G2R | GTCATYTGDGTDGCDGGYTCDCC | O’Donnell et al. (2010) | ||
| RPB2 | 5F2 | GGGGWGAYCAGAAGAAGGC | 94 °C to 90 s; Cycles of 94 °C 45 s, 58 °C 45 s, 72 °C 2 min; Cycles of 94 °C 45 s, 57 °C 45 s, 72 °C 2 min; Cycles of 94 °C 45 s, 56 °C 45 s, 72 °C 2 min; 72 °C for 10 min; Soak 10 °C | O’Donnell et al. (2010) |
| 7CR | CCCATRGCTTGYTTRCCCAT | O’Donnell et al. (2010) | ||
Note:
The total volume of the PCR mixture was 50 μL (Lombard, Van & Crous, 2019), containing 19 μL double-distilled water, 2 μL genomic DNA, 2 μL of each primer, and 25 μL Taq DNA polymerase mix. After PCR, the products were sent to Shanghai Jieli Biotechnology Co., Ltd. for DNA sequencing. All sequences with primers CL1/CL2A, FA/G2R, 5F2/7CR, and EF1/EF2 of ZE-1b was deposited in GenBank under accession numbers MW890756, MZ146450, MW890757, MZ088053, and ZE-2b was deposited in GenBank under accession numbers MW885175, MZ127817, MZ126726 and MW885176, respectively.
The CAMD, RPB1, RPB2, and TEF1 sequences were compared to sequences in GenBank using BLAST. The sequences were obtained from GenBank for phylogenetic analyses (Table 2). We downloaded sequences for which the comparison results showed higher than 99% similarity. Using Fusarium aywerte as the outgroup. The arrangement of each gene/region was compared with MAFFTver.7.313 (Katoh & Standley, 2013) and manually adjusted with BioEditver.7.0 (Hall, 1999). It was a combination of these four genes/regions. The ModelFinder was used to select the best-fit model (Kalyaanamoorthy et al., 2017). In IQTree ver.1.6.8, the alternative model of GTR + F + I + G4 was adopted, 1,000 iteration guidance methods were used, and the maximum-likelihood ground method (ML) analysis was used to estimate the system relationship (Nguyen et al., 2015). In the GTR + I + G + F model (2 parallel runs, 2 million generations), MRBayesver.3.2.6 was used for Bayesian analysis. Using burn-in, 25% of sampled data were discard (Ronquist et al., 2012). The phylogenetic trees were drawn with FigTree ver. 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
| GenBank accession | |||||||
|---|---|---|---|---|---|---|---|
| Species | Culture accession | Host/substrate | Origin | CAMD | RPB1 | RPB2 | TEF1 |
| F. acacia-mearnsii | NRRL 26755 = CBS 110255 = MRC 5122 | Acacia mearnsii | South Africa | – | KM361640 | KM361658 | AF212449 |
| F. armeniacum | NRRL 43641 | Horse eye | USA | GQ505398 | HM347192 | GQ505494 | GQ505430 |
| F. asiaticum | NRRL 13818 = CBS110257 = FRC R-5469 = MRC 1963 = NRRL 31547T | Hordeum vulgare | Japan | - | JX171459 | JX171573 | AF212451 |
| F. atrovinosum | NRRL 34013 | Human toenail | USA | GQ505378 | – | GQ505472 | GQ505408 |
| NRRL 34015 | Human eye | USA | GQ505380 | – | GQ505474 | GQ505410 | |
| F. aywerte | NRRL 25410T | Soil | Australia | KU171417 | JX171513 | JX171626 | KU171717 |
| F. boothii | NRRL 26916 = ATCC 24373 = CBS 316.73 = NRRL 26855T | Zea mays | South Africa | - | KM361641 | KM361659 | AF212444 |
| F. brachygibbosum | NRRL 34033 | Human foot | USA | GQ505388 | HM347172 | GQ505482 | GQ505418 |
| F. cerealis | NRRL 25491 = CBS 589.93 | Iris hollandica | Netherlands | – | MG282371 | MG282400 | AF212465 |
| F. chlamydosporum | CBS 145.25 = NRRL 26912NT | Musa sapientum | Honduras | MN120695 | MN120715 | MN120735 | MN120754 |
| CBS 615.87 = NRRL 28578 | Colocasia esculenta | Cuba | GQ505375 | JX171526 | GQ505469 | GQ505405 | |
| F. coffeatum |
CBS 635.76 = BBA 62053 = NRRL 20841T |
Cynodon lemfuensis | South Africa | MN120696 | MN120717 | MN120736 | MN120755 |
| CBS 430.81 = NRRL 28577 | Grave stone | Romania | MN120697 | – | MN120737 | MN120756 | |
| F. culmorum | NRRL 25475 = CBS 417.86 = FRC R-8504 = IMI 309344 |
Hordeum vulgare | Denmark | - | JX171515 | JX171628 | AF212463 |
| F. graminearum | NRRL 36905 | Triticum aestivum | USA | – | KM361646 | KM361664 | DQ459742 |
| F. humicola | CBS 124.73 = NRRL 25535T | Soil | Pakistan | MN120698 | MN120718 | MN120738 | MN120757 |
| F. lacertarum | NRRL 20423 = ATCC 42771 = CBS 130185 = IMI 300797T | Lizard skin | India | GQ505505 | JX171467 | JX171581 | GQ505593 |
| CBS 127131 | Soil | USA | MN120699 | MN120720 | MN120739 | MN120758 | |
| NRRL 43680 | Contact lens fluid | USA | – | – | EF470046 | EF453007 | |
| F. langsethiae | NRRL 53409 | Hordeum vulgare | Finland | – | – | HQ154455 | HM744667 |
| F. lunulosporum |
NRRL 13393 = BBA 62459 = CBS 636.76 = FRC R-5822 = IMI 322097T |
Citrus paradisi | South Africa | - | KM361637 | KM361655 | AF212467 |
| F. microconidium | CBS 119843 = MRC 839 | Unknown | Unknown | MN120700 | MN120721 | – | MN120759 |
| F. nelsonii |
CBS 119876 = FRC R 8670 = MRC 4570T |
Plant debris | South Africa | MN120701 | MN120722 | MN120740 | MN120760 |
| F. nodosum | CBS 200.63 | Arachis hypogaea | Portugal | MN120703 | MN120724 | MN120742 | MN120762 |
| CBS 201.63T | Arachis hypogaea | Portugal | MN120704 | MN120725 | MN120743 | MN120763 | |
| F. oxysporum | CBS 144143T | Solanum tuberosum | Germany | MH484771 | - | MH484953 | MH485044 |
| CFCC 55679 = ZE-1b* | Hosta ventricosa | China | MW890756 | MZ146450 | MW890757 | MZ088053 | |
| CFCC 55680 = ZE-2b* | Hosta ventricosa | China | MW885175 | MZ127817 | MZ126726 | MW885176 | |
| F. peruvianum | CBS 511.75T | Gossypium sp. | Peru | MN120707 | MN120728 | MN120746 | MN120767 |
| F. poae | NRRL 66297 | – | – | MG282363 | MG282392 | – | |
| F. pseudograminearum |
NRRL 28062 = CBS 109956 = FRCR 5291 = MAFF 237835T |
Hordeum vulgare | Australia | - | JX171524 | JX171637 | AF212468 |
| F. sibiricum | NRRL 53429 | Avena sativa | Russia | – | – | HQ154471 | HM744683 |
| NRRL 53430T | Avena sativa | Russia | - | - | HQ154472 | HM744684 | |
| F. sporodochiale | CBS 199.63= MUCL 6771 | Termitary | Unknow | MN120709 | MN120730 | MN120748 | MN120769 |
|
CBS 220.61 = ATCC 14167 = NRRL 20842T |
Soil | South Africa | MN120710 | MN120731 | MN120749 | MN120770 | |
| F. sporotrichioides | CBS 462.94 | Glycosmis citrifolia | Austria | MN120711 | MN120732 | MN120750 | MN120771 |
| FIESC 24 | CBS 101138 = BBA 70869 | Phaseolus vulgaris | Turkey | MN120712 | MN120733 | MN120751 | MN120772 |
| Fusarium sp. | NRRL 13338 | Soil | Australia | GQ505372 | JX171447 | JX171561 | GQ505402 |
Results
Incidence of disease and symptoms
The incidence of leaf spot of H. ventricosa in three areas of Nanjing was investigated, and the results showed that the incidence of leaf spot of H. ventricosa in the field was 40%. When the H. ventricosa leaves were infected, the edge of leaves will turn green and yellow, and be dull. With the development of disease, the leaf spots extended and gradually turned yellowish brown.
Pathogenicity of fungal isolates
Based on the colony morphology, fifty fungal samples were divided into seven types. More than 50% are classified as ZE-1b/ZE-2b types. According to the colony morphology, fungi were divided into seven kinds namely ZE-a–ZE-g. According to the ITS sequence ZE-a–ZE-g were identified as Fusarium oxysporum (50%), Fusarium ipomoeae (20%), Fusarium equsiti (10%), Colletotrichum spaethianum (9%), Nigrospora spherica (5%), Colletotrichum gloeosporioide (4%), Colletotrichum siamense (2%). All of the seven kinds of isolates were inoculated seedings, replicated 3 times.
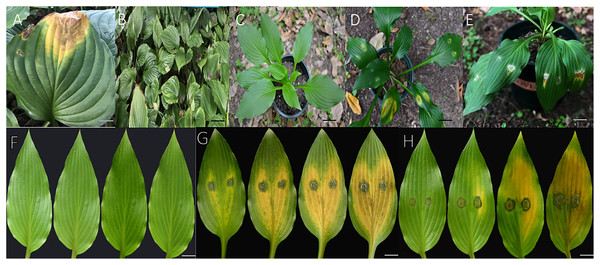
Inoculated H. ventricosa showed leaf spot disease consistent with that observed previously. Two isolates (ZE-1b and ZE-2b) were proven pathogenic to H. ventricosa leaves. Lesions appeared on detached leaves 3 days after inoculation using mycelial plugs (Figs. 1G and 1H). In live plants, 1 week after inoculation, the leaves began to show obvious symptoms of infection, turning yellow and withering (Figs. 1D and 1E). In addition, no lesions were observed on leaves from the control plants (Cong, 2017) (Figs. 1C, 1F). The symptoms on detached leaves and live plants after inoculation were the same as those in the field (Figs. 1A and 1B). The reisolated pathogens from inoculated diseased leaves were consistent with those obtained in the first isolation. Therefore, it was determined that ZE-1b and ZE-2b were the main pathogens causing H. ventricosa leaf spot.
Figure 1: Pathogenicity in detached leaves and in live plants.
(A and B) Diseased leaves naturally infected. (C) No symptoms were observed on leaves from control plants 7 days after inoculation with sterile water; (D) symptoms on live leaves 7 days after inoculation with mycelial plugs of ZE-1b; (E) Symptoms on live leaves 7 days after inoculation with mycelial plugs of ZE-2b; (F) no symptoms were observed on detached leaves from control plants 3, 5, 7 and 10 days after inoculation with sterile water; (G) symptoms on detached leaves 3, 5, 7 and 10 days after inoculation with mycelial plugs of ZE-1b; (H) symptoms on detached leaves 3, 5, 7 and 10 days after inoculation with mycelial plugs of ZE-2b; Bars A = 2 cm; B = 5 cm; C–E = 3 cm; F–H = 1 cm.Morphological characteristics of fungal isolates
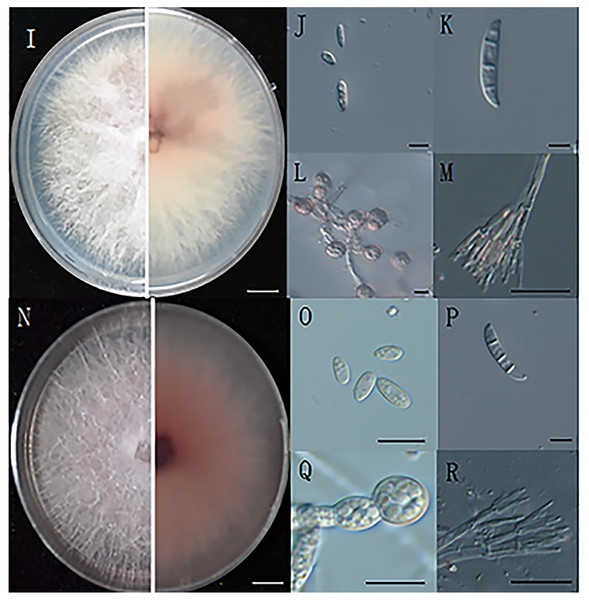
Morphological observations of the pathogenic fungi were carried out. Colonies were inoculated on PDA plates and cultured at 25 °C for 4 days, and the colony diameter was 7 cm (Figs. 2I, 2N). The hyphae grew radially, luxuriously and densely, and the aerial hyphae were velvety, white or pink-white (Liu et al., 2020).
Figure 2: The morphology of hyphae and conidia.
(I) The front and reverse colony morphology of ZE-1b; (J) microconidia of ZE-1b; (K) macroconidia of ZE-1b; (L) chlamydospore formation of ZE-1b; (M) conidiophores of ZE-1b; (N) front and reverse colony morphology of ZE-2b; (O) microconidia of ZE-2b; (P) macroconidia of ZE-2b; (Q) chlamydospore formation of ZE-2b; (R) conidiophores of ZE-2b; Bars I, N = 1 cm; J–M, O–R = 10 m.Fusarium has three types of conidia for reproduction and survival under adverse environments: microconidia, macroconidia, and chlamydospores. Microconidia were numerous, oval or kidney-shaped, and scattered, with the size of 4.7–8.6 μm × 2.5–4.7μm (Figs. 2J, 2O). Macroconidia were sickle-shaped, generally symmetrical, slightly curved, and tapering toward the ends, with the size of 23–50.6 μm × 3–5 μm (Figs. 2K, 2P). Chlamydospores were readily produced, with smooth and spherical surfaces (Figs. 2L, 2Q). They were solitary, paired or clustered between hyphae (Du, 2017).
Phylogenetic analyses
Sequences of the genes/regions CAMD, RPB1, RPB2, and TEF1 from the two isolates (ZE-1b and ZE-2b) were deposited in GenBank, and the accession numbers are shown in Table 2. The sequences from ZE-1b and ZE-2b showed 100% similarity with F. oxysporum. These results further indicate that isolation, purification, morphological identification and molecular biology can be used in combination for accurate and reliable results (Cong, 2017).
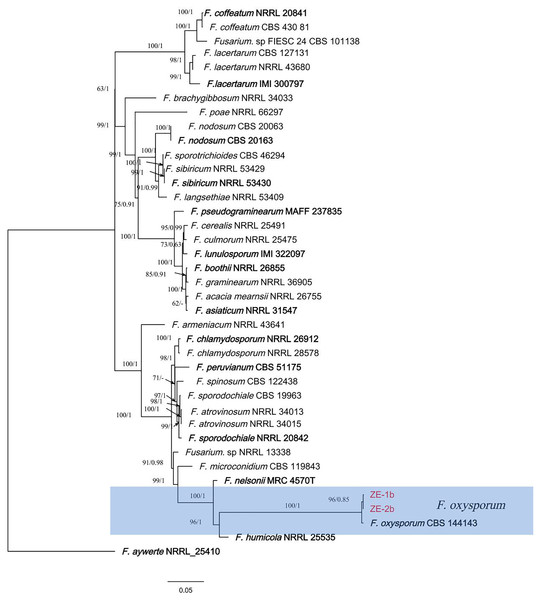
In the ML phylogenetic tree, two isolates (ZE-1b and ZE-2b) were in the same cluster as F. oxysporum with 100% RAxML bootstrap support values (Fig. 3). The phylogenetic tree obtained by Bayesian analysis was consistent with the ML tree. Bayesian analyses showed that the isolates clustered with F. oxysporum with a high Bayesian posterior probability. Two isolates (ZE-1b and ZE-2b) were identified as F. oxysporum based on multigene phylogeny and morphology.
Figure 3: A maximum parsimony phylogeny for Fusarium oxysporum.
Phylogenetic relationship of ZE-1b and ZE-2b with related taxa derived from maxmum-likelihood (ML) analysis using combined CAMD, RPB1, RPB2, and TEF1 sequence alignment of Fusarium spp., With Fusarium aywerte (NRRL 25410) as the outgroup. RAxML bootstrap support values (ML ≥ 50) and Bayesian posterior probability (PP ≥ 0.80) are shown at the nodes (ML/PP). Ex-type strains are marked in bold. Isolates from H. ventricosa marked in red.Discussion
In this study, a novel leaf spot disease was studied through pathogenicity determination, morphological identification, and molecular biological identification, and the results showed that the pathogen was F. oxysporum. Herein, wilt of H. ventricosa leaves caused by F. oxysporum was reported for the first time in China.
F. oxysporum is a facultative parasitic fungus that can both infect plants and live in soil (Yang et al., 2021b; Cong, 2017). The transmission of the isolate is either vertical transmission through the mother line to the next generation of seeds or horizontal transmission when the fungi in soil or crops infects the host through wounds. The main means of horizontal transmission are as follows: fungal isolates infect and destroy the vascular bundle from the roots (Foley, 1962) and stems of the plant and spread to various parts of the plant. Due to the exposure of stomata and other external tissues of crops as well as plant wounds, spore and mycelial infection via the air can also occur (Headrick, 1991). Most Fusarium enter through natural openings in plants or seeds, such as stomata (Lin et al., 2014).
F. oxysporum is highly destructive and can destroy many plant organs and cause very severe diseases, such as leaf spot, root rot, stem rot, flower rot and grain wilt (Liu et al., 2020). Globally, F. oxysporum has been identified as a wilt pathogen in many host plants, such as bananas (Maryani et al., 2019; Forsyth, Smith & Aitken, 2006), cotton (Xie et al., 2020; Davis et al., 2006; Zhu et al., 2020), cucumbers (Jaber et al., 2020), sesame (Khalifa, 1997), grapes (El-Sayed, El-Sayed & Eman, 2011), basil (Lori, Malbran & Mourelos, 2014; Mamta et al., 2013; Salim, Salman & Jasim, 2017; Basco et al., 2017), lettuce (Guerrero et al., 2020) and pecan (Rolim et al., 2020). Leaves wilt and eventually drop to the ground, leading to a large area of growth decline; at worst, the whole plant winters and dies, which eventually leads to reductions in yield and quality, causing huge economic losses (Yang et al., 2021a; Li et al., 2020; Cong, 2017).
Originally by scientists abroad, Fusarium was considered a crescent-shaped fungus born on the seed coat. Because many other fungi also produce such sickle-like spores and fungal culture techniques have limitations, the classification of Fusarium has long been in a state of confusion. Later, German scientists introduced the first systematic classification of Fusarium and proposed a relatively complete classification system based on the biological characteristics of these fungi, combined with their morphological structures, which laid the foundation for classification research on Fusarium (Du, 2017). Fusarium was initially divided into 44 strains, with 35 strains in China, laying a foundation for the study of Fusarium here (Yu, 1977). Currently, more than 3,000 strains of Fusarium have been studied, 40 physiological strains have been identified and collected, and one new strain was found. Twenty-eight strains of Fusarium zhejiangensis were identified in Zhejiang, and Fusarium zhejiangensis was first recorded in literature. “Fusarium disease in Taiwan” was published in Plant Pathology, Chung Hsing University, Taiwan (Du, 2017).
In recent years, research on Fusarium taxonomy in China has developed rapidly based on both morphology and molecular biology. This experiment provides a basis for field prevention and treatment of H. ventricosa leaf spot caused by Fusarium and provides a reference for further genetic analysis and cultivation of disease resistant varieties of H. ventricosa (Zhi, 2020).
Conclusion
This is the first report of H. ventricosa leaf spot in China and Chinese H. ventricosa is a new host of F. oxysporum. We should take reasonable preventive measures against diseases. This study provided theoretical guidance for the control of Chinese H. ventricosa leaf spot.