The Role of the NLRP3 Inflammasome in the Molecular and Biochemical Mechanisms of Cervical Ripening: A Comprehensive Review
Abstract
:1. Introduction
2. Uterine Cervix Biology
3. NLRP3 Inflammasome Biology
3.1. NLRP3 Inflammasome Structure
3.2. NLRP3 Inflammasome Activation and Regulation
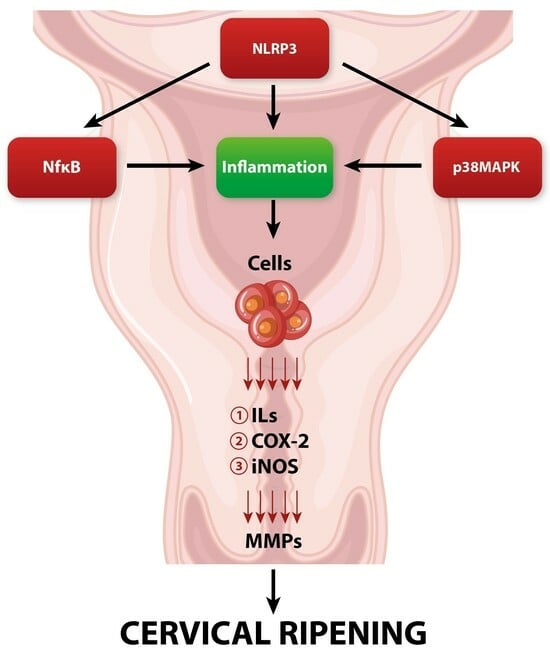
4. Inflammatory Response and NLRP3 Inflammasome during Cervical Remodeling
4.1. Inflammation during Cervical Ripening and Its Regulatory Factors
4.2. NLRP3 Inflammasome in the Cervical Inflammatory Response
5. NLRP3 Inflammasome and Reactive Oxygen and Nitrogen Species in Cervical Tissue
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martyn, F.; McAuliffe, F.M.; Wingfield, M. The role of the cervix in fertility: Is it time for a reappraisal? Hum. Reprod. 2014, 29, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Sennström, M.B.; Ekman, G.; Westergren-Thorsson, G.; Malmström, A.; Byström, B.; Endrésen, U.; Mlambo, N.; Norman, M.; Ståbi, B. Annelie Brauner, Human cervical ripening, an inflammatory process mediated by cytokines. Mol. Hum. Reprod. 2000, 6, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ekman-Ordeberg, G.; Stjernholm, Y.; Wang, H.; Stygar, D.; Sahlin, L. Endocrine regulation of cervical ripening in humans—Potential roles for gonadal steroids and insulin-like growth factor-I. Steroids 2003, 68, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Plazyo, O.; Unkel, R.; Leng, Y.; Than, N.G.; Chaiworapongsa, T.; Panaitescu, B.; Dong, Z.; et al. A Role for the Inflammasome in Spontaneous Preterm Labor with Acute Histologic Chorioamnionitis. Reprod. Sci. 2017, 24, 1382–1401. [Google Scholar] [CrossRef]
- Spencer, T.E.; Hayashi, K.; Hu, J.; Carpenter, K.D. Comparative developmental biology of the mammalian uterus. Curr. Top. Dev. Biol. 2005, 68, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.M.; Feltovich, H.; Mazza, E.; Vink, J.; Bajka, M.; Wapner, R.J.; Hall, T.J.; House, M. The mechanical role of the cervix in pregnancy. J. Biomech. 2015, 48, 1511–1523. [Google Scholar] [CrossRef]
- Leppert, P.C. Anatomy and physiology of cervical ripening. Clin. Obstet. Gynecol. 1995, 38, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.Y.; Qin, S.; Brock, C.O.; Zork, N.M.; Feltovich, H.M.; Chen, X.; Urie, P.; Myers, K.M.; Hall, T.J.; Wapner, R.; et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am. J. Obstet. Gynecol. 2016, 215, 478.e1–478.e11. [Google Scholar] [CrossRef]
- Nicoll, A. The Physiology of Cervical Ripening and the Induction of Labour: A Potential Role for the Nitric Oxide Donor Isosorbide Mononitrate; University of Glasgow: Glasgow, UK, 2001. [Google Scholar]
- Iwahashi, M.; Muragaki, Y.; Ooshima, A.; Umesaki, N. Decreased type I collagen expression in human uterine cervix during pregnancy. J. Clin. Endocrinol. Metab. 2003, 88, 2231–2235. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yao, W.; Harris, W.B.R.; Vink, J.Y.; Myers, K.M.; Donnelly, E. Characterization of the collagen microstructural organization of human cervical tissue. Reproduction 2018, 156, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Oxlund, B.S.; Ørtoft, G.; Brüel, A.; Danielsen, C.C.; Bor, P.; Oxlund, H.; Uldbjerg, N. Collagen concentration and biomechanical properties of samples from the lower uterine cervix in relation to age and parity in non-pregnant women. Reprod. Biol. Endocrinol. 2010, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Halper, J. Proteoglycans and diseases of soft tissues. Adv. Exp. Med. Biol. 2014, 802, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Akgul, Y.; Holt, R.; Mummert, M.; Word, A.; Mahendroo, M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology 2012, 153, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Jayyosi, C.; Lee, N.; Mahendroo, M.; Myers, K.M. Mechanics of cervical remodelling: Insights from rodent models of pregnancy. Interface Focus 2019, 9, 20190026. [Google Scholar] [CrossRef] [PubMed]
- Ruscheinsky, M.; De la Motte, C.; Mahendroo, M. Hyaluronan and its binding proteins during cervical ripening and parturition: Dynamic changes in size, distribution and temporal sequence. Matrix Biol. 2008, 27, 487–497. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, S.; Yoshida, K.; Akins, M.; Myers, K.; Iozzo, R.; Mahendroo, M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers. Endocrinology 2017, 158, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Keller, S.; Cerreta, J.; Hosannah, Y.; Mandl, I. The content of elastin in the uterine cervix. Arch. Biochem. Biophys. 1983, 222, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.D. Cervical ripening: Why we do what we do. Semin. Perinatol. 2020, 44, 151216. [Google Scholar] [CrossRef]
- Wheeler, V.; Hoffman, A.; Bybel, M. Cervical Ripening and Induction of Labor. Am. Fam. Physician 2022, 105, 177–186, Erratum in Am. Fam. Physician 2022, 106, 121. [Google Scholar] [PubMed]
- El Maradny, E.; Kanayama, N.; Kobayashi, H.; Hossain, B.; Khatun, S.; Liping, S.; Kobayashi, T.; Terao, T. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum. Reprod. 1997, 12, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Uldbjerg, N.; Ekman, G.; Malmström, A.; Olsson, K.; Ulmsten, U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am. J. Obstet. Gynecol. 1983, 147, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Di, X.; Wang, L.; Zhang, W.; Feng, Y.; Shi, S.Q.; Garfield, R.E.; Liu, H. Evaluation of cervical maturity by cervical collagen measurement using light-induced fluorescence (LIF) during pregnancy. J. Int. Med. Res. 2020, 48, 300060520964006. [Google Scholar] [CrossRef] [PubMed]
- Maul, H.; Mackay, L.; Garfield, R.E. Cervical ripening: Biochemical, molecular, and clinical considerations. Clin. Obstet. Gynecol. 2006, 49, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Sennström, M.B.; Brauner, A.; Byström, B.; Malmström, A.; Ekman, G. Matrix metalloproteinase-8 correlates with the cervical ripening process in humans. Acta Obstet. Gynecol. Scand. 2003, 82, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Stygar, D.; Wang, H.; Vladic, Y.S.; Ekman, G.; Eriksson, H.; Sahlin, L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol. Reprod. 2002, 67, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Jung, K.L.; Oh, S.Y.; Kim, J.H.; Roh, C.R. Cervicovaginal matrix metalloproteinase-9 and cervical ripening in human term parturition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 142, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Mao, Q.X.; Luo, J.H. Dynamic expression of matrix metalloproteinases 2 and 8 in rat cervix. Nan Fang Yi Ke Da Xue Xue Bao 2009, 29, 2205–2207. (In Chinese) [Google Scholar] [PubMed]
- Bollapragada, S.; Youssef, R.; Jordan, F.; Greer, I.; Norman, J.; Nelson, S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol. 2009, 200, 104.e1–104.e11, Erratum in Am. J. Obstet. Gynecol. 2009, 201, 214. Bollopragada, Shrikant [corrected to Bollapragada, Shrikant]. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.C.; Li, S.; Liang, K.; Ting, J.P. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell 2023, 186, 2288–2312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.G.; Dash, P.; Aldridge, J.R., Jr.; Ellebedy, A.H.; Reynolds, C.; Funk, A.J.; Martin, W.J.; Lamkanfi, M.; Webby, R.J.; Boyd, K.L.; et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009, 30, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Poeck, H.; Bscheider, M.; Dostert, C.; Hannesschläger, N.; Endres, S.; Hartmann, G.; Tardivel, A.; Schweighoffer, E.; Tybulewicz, V.; et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Oroz, J.; Barrera-Vilarmau, S.; Alfonso, C.; Rivas, G.; de Alba, E. ASC Pyrin Domain Self-associates and Binds NLRP3 Protein Using Equivalent Binding Interfaces. J. Biol. Chem. 2016, 291, 19487–19501. [Google Scholar] [CrossRef] [PubMed]
- Manji, G.A.; Wang, L.; Geddes, B.J.; Brown, M.; Merriam, S.; Al-Garawi, A.; Mak, S.; Lora, J.M.; Briskin, M.; Jurman, M.; et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J. Biol. Chem. 2002, 277, 11570–11575. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Menu, P.; Vince, J.E. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Kanneganti, T.D. Nlrp3: An immune sensor of cellular stress and infection. Int. J. Biochem. Cell Biol. 2010, 42, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Warner, N.; Viani, K.; Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Bergstralh, D.T.; Wang, Y.; Willingham, S.B.; Ye, Z.; Zimmermann, A.G.; Ting, J.P. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 8041–8046. [Google Scholar] [CrossRef] [PubMed]
- Proell, M.; Gerlic, M.; Mace, P.D.; Reed, J.C.; Riedl, S.J. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem. J. 2013, 449, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Elizagaray, M.L.; Gomes, M.T.R.; Guimaraes, E.S.; Rumbo, M.; Hozbor, D.F.; Oliveira, S.C.; Moreno, G. Canonical and Non-canonical Inflammasome Activation by Outer Membrane Vesicles Derived from Bordetella pertussis. Front. Immunol. 2020, 11, 1879. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Anand, P.K.; Malireddi, R.K.; Vande Walle, L.; Van Opdenbosch, N.; Dillon, C.P.; Weinlich, R.; Green, D.R.; Lamkanfi, M.; Kanneganti, T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014, 192, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, Q.; Xu, G.; Xiao, X.; Bai, Z. The mechanism of NLRP3 inflammasome activation and its pharmacological inhibitors. Front. Immunol. 2023, 13, 1109938. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Zhang, Y.; Li, Y.; Kwak-Kim, J.; Wu, L. NLRP3 Inflammasome and Its Critical Role in Gynecological Disorders and Obstetrical Complications. Front. Immunol. 2021, 11, 555826. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Ismail, N. Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells 2023, 12, 2597. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz Garcia, A.B.; Schnur, K.P.; Malik, A.B.; Mo, G.C.H. Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Devant, P.; Kagan, J.C. Molecular mechanisms of gasdermin D pore-forming activity. Nat. Immunol. 2023, 24, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrod, T.; Dalpke, A.H. Bacterial RNA: An Underestimated Stimulus for Innate Immune Responses. J. Immunol. 2015, 195, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Ghosh, S.; Monks, B.; DeOliveira, R.B.; Tzeng, T.C.; Kalantari, P.; Nandy, A.; Bhattacharjee, B.; Chan, J.; Ferreira, F.; et al. RNA and β-hemolysin of group B Streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. J. Biol. Chem. 2014, 289, 13701–13705. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Mitoma, H.; Hanabuchi, S.; Bao, M.; Weng, L.; Sugimoto, N.; Liu, Y.; Zhang, Z.; Zhong, J.; Sun, B.; et al. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc. Natl. Acad. Sci. USA 2014, 111, 16059–16064. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; Apolloni, S.; Skaper, S.D.; Burnstock, G. P2X7 receptors: Channels, pores and more. CNS Neurol. Disord. Drug Targets 2012, 11, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Ozkurede, U.; Kim, Y.G.; Arindam, C.; Gale MJr Silverman, R.H.; Colonna, M.; Akira, S.; Núñez, G. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J. Immunol. 2014, 193, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Burgk, J.L.; Chauhan, D.; Schmidt, T.; Ebert, T.S.; Reinhardt, J.; Endl, E.; Hornung, V. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Y.; Li, X.; Zhan, X.; Tang, M.; Fina, M.; Su, L.; Pratt, D.; Bu, C.H.; Hildebrand, S.; et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 2016, 17, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, L.; Li, L. NEK7: A novel promising therapy target for NLRP3-related inflammatory diseases. Acta Biochim. Biophys. Sin. 2016, 48, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Lang, X.; Xu, C.; Wang, X.; Gong, T.; Yang, Y.; Cui, J.; Bai, L.; Wang, J.; Jiang, W.; et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Yang, Y.; Jin, T.; Jiang, W.; Zhou, R. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol. 2018, 39, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schöneberg, T.; Schaefer, M.; Krügel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.H.; Carroll, R.G.; Wei, P.; Jones, K.A.; Rice, A.P. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J. Virol. 1998, 72, 9881–9888. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Saharinen, P.; Pesu, M.; Holt, V.E., 3rd; Silvennoinen, O.; O’Shea, J.J. The Janus kinases (Jaks). Genome Biol. 2004, 5, 253. [Google Scholar] [CrossRef]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, P.A.; Kertesy, S.B.; Lundberg, K.; Kahlenberg, J.M.; Dubyak, G.R. Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J. Immunol. 2005, 175, 7623–7634. [Google Scholar] [CrossRef] [PubMed]
- Perregaux, D.G.; Laliberte, R.E.; Gabel, C.A. Human monocyte interleukin-1beta posttranslational processing. Evidence of a volume-regulated response. J. Biol. Chem. 1996, 271, 29830–29838. [Google Scholar] [CrossRef] [PubMed]
- Compan, V.; Baroja-Mazo, A.; López-Castejón, G.; Gomez, A.I.; Martínez, C.M.; Angosto, D.; Montero, M.T.; Herranz, A.S.; Bazán, E.; Reimers, D.; et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012, 37, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Fernández, R.; Coll, R.C.; Kearney, J.; Breit, S.; O’Neill, L.A.J. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1β transcription and activate the NLRP3 inflammasome. J. Biol. Chem. 2017, 292, 12077–12087. [Google Scholar] [CrossRef] [PubMed]
- Green, J.P.; Yu, S.; Martín-Sánchez, F.; Pelegrin, P.; Lopez-Castejon, G.; Lawrence, C.B.; Brough, D. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc. Natl. Acad. Sci. USA 2018, 115, E9371–E9380. [Google Scholar] [CrossRef] [PubMed]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Boucher, D.; Bierschenk, D.; Tebartz, C.; Whitney, P.G.; D’Silva, D.B.; Tanzer, M.C.; Monteleone, M.; Robertson, A.A.; Cooper, M.A.; et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 2015, 45, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Miura, M.; Jung, Y.K.; Zhu, H.; Li, E.; Yuan, J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 1998, 92, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Rühl, S.; Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Guilbert, L.J.; Olson, D.M. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J. Leukoc. Biol. 2010, 88, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Stjernholm-Vladic, Y.; Stygar, D.; Mansson, C.; Masironi, B.; Akerberg, S.; Wang, H.; Ekman-Ordeberg, G.; Sahlin, L. Factors involved in the inflammatory events of cervical ripening in humans. Reprod. Biol. Endocrinol. 2004, 2, 74. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W. Inflammatory mediators and cervical ripening. J. Reprod. Immunol. 2002, 57, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Shuai, H.; Luo, X.; Wang, X.; Guan, B. The clinical and prognostic value of CXCL8 in cervical carcinoma patients: Immunohistochemical analysis. Biosci. Rep. 2017, 37, BSR20171021. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.Y.; Goetzl, L.; Unal, R.; Cierny, J.; Flood, P. The Relationship between Plasma Inflammatory Cytokines and Labor Pain. Anesth. Analg. 2015, 121, 748–751. [Google Scholar] [CrossRef] [PubMed]
- el Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Sumimoto, K.; Terao, T. The effect of interleukin-1 in rabbit cervical ripening. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 60, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Watari, M.; Watari, H.; DiSanto, M.E.; Chacko, S.; Shi, G.P.; Strauss, J.F., 3rd. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am. J. Pathol. 1999, 154, 1755–1762. [Google Scholar] [CrossRef]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 Across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef]
- Ristimäki, A.; Garfinkel, S.; Wessendorf, J.; Maciag, T.; Hla, T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J. Biol. Chem. 1994, 269, 11769–11775. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.; Kubota, Y.; Yamashiro, T.; Takeuchi, H.; Ninomiya, T.; Suyama, Y.; Shirasuna, K. Signaling pathways regulating IL-1alpha-induced COX-2 expression. J. Dent. Res. 2007, 86, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Mertin, J.; Stackpoole, A.; Shumway, S.J. Prostaglandins and cell-mediated immunity. The role of prostaglandin E1 in the induction of host-versus-graft and graft-versus-host reactions in mice. Transplantation 1984, 37, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; DeWitt, D.L.; McNeely, T.B.; Wahl, S.M.; Wahl, L.M. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J. Clin. Investig. 1997, 99, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.D.; Kashiwagi, K.; Boyle, D.; Kashiwagi, F.; Firestein, G.S.; Weinreb, R.N. Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr. Eye Res. 1996, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Karli, S.; Ayala-Haedo, J.A.; Feuer, W.J.; Fernandez, M.; Dubovy, S.; Wester, S.T. Effect of prostaglandin analogs on matrix metalloproteinases and tissue inhibitor of metalloproteinases in eyelid muscle specimens. Clin. Ophthalmol. 2018, 12, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.A.; Mitchell, B.F.; Olson, D.M. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 2008, 79, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, V.; Mortazavi, M.; Ghorban, K.; Dadmanesh, M.; Bahramabadi, R.; Rezayati, M.T.; Javadi-Moghadam, E.; Rezaei, Z.; Sabzali, Z.; Fatemi, I.; et al. Role of maternal interleukin-8 (IL-8) in normal-term birth in the human. Reprod. Fertil. Dev. 2019, 31, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Osmers, R.G.; Bläser, J.; Kuhn, W.; Tschesche, H. Interleukin-8 synthesis and the onset of labor. Obstet. Gynecol. 1995, 86, 223–229. [Google Scholar] [CrossRef]
- Winkler, M.; Fischer, D.C.; Hlubek, M.; van de Leur, E.; Haubeck, H.D.; Rath, W. Interleukin-1beta and interleukin-8 concentrations in the lower uterine segment during parturition at term. Obstet. Gynecol. 1998, 91, 945–949. [Google Scholar] [CrossRef]
- Khatun, S.; Kanayama, N.; Md Belayet, H.; Yonezawa, M.; Kobayashi, T.; Terao, T. Interleukin-8 potentiates the effect of interleukin-1-induced uterine contractions. Hum. Reprod. 1999, 14, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Lattey, K.R.; Kelly, A.J. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database Syst. Rev. 2016, 12, CD006901. [Google Scholar] [CrossRef] [PubMed]
- Tschugguel, W.; Schneeberger, C.; Lass, H.; Stonek, F.; Zaghlula, M.B.; Czerwenka, K.; Schatten, C.; Kaider, A.; Husslein, P.; Huber, J.C. Human cervical ripening is associated with an increase in cervical inducible nitric oxide synthase expression. Biol. Reprod. 1999, 60, 1367–1372. [Google Scholar] [CrossRef]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Chwalisz, K.; Shao-Qing, S.; Garfield, R.E.; Beier, H.M. Cervical ripening in guinea-pigs after a local application of nitric oxide. Hum. Reprod. 1997, 12, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, G.M.; Giampietro, F. Inhibition of nitric oxide synthesis causes preterm delivery in the mouse. Hum. Reprod. 2000, 15, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Väisänen-Tommiska, M.R. Nitric oxide in the human uterine cervix: Endogenous ripening factor. Ann. Med. 2008, 40, 45–55. [Google Scholar] [CrossRef]
- Sasaki, K.; Hattori, T.; Fujisawa, T.; Takahashi, K.; Inoue, H.; Takigawa, M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J. Biochem. 1998, 123, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, C.C.; Madara, P.J.; Van Dervort, A.L.; Tropea, M.M.; Wesley, R.A.; Danner, R.L. Effects of nitric oxide on chemotaxis and endotoxin-induced interleukin-8 production in human neutrophils. J. Infect. Dis. 1998, 177, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer. 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Ledingham, M.A.; Denison, F.C.; Kelly, R.W.; Young, A.; Norman, J.E. Nitric oxide donors stimulate prostaglandin F(2alpha) and inhibit thromboxane B(2) production in the human cervix during the first trimester of pregnancy. Mol. Hum. Reprod. 1999, 5, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A. Nitric Oxide Synthase and Cyclooxygenase Pathways: A Complex Interplay in Cellular Signaling. Curr. Med. Chem. 2016, 23, 2559–2578. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Filipovich, Y.; Mahendroo, M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am. J. Obstet. Gynecol. 2006, 194, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Kim, H.; Kim, K.H. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab. Investig. 2001, 81, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Allport, V.C.; Pieber, D.; Slater, D.M.; Newton, R.; White, J.O.; Bennett, P.R. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol. Hum. Reprod. 2001, 7, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Madrid, L.V.; Wang, C.Y.; Guttridge, D.C.; Schottelius, A.J.; Baldwin, A.S., Jr.; Mayo, M.W. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol. Cell. Biol. 2000, 20, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.M.; Gomez, P.F.; Abramson, S.B. Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthr. Cartil. 2004, 12, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B.; Cameron, P.M.; O’Keefe, S.J.; Frantz-Wattley, B.; Thompson, J.; O’Neill, E.A.; Tennis, T.; Liu, L.; Becker, J.W.; Scapin, G. The three-dimensional structure of MAP kinase p38beta: Different features of the ATP-binding site in p38beta compared with p38alpha. Acta Crystallogr. Sect. D Struct. Biol. 2009, 65, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Stjernholm, Y.V. Plasma membrane receptor mediated MAPK signaling pathways are activated in human uterine cervix at parturition. Reprod. Biol. Endocrinol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Spasic-Boskovic, O.; Jauniaux, E.; Charnock-Jones, D.S.; Burton, G.J. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: Effects of antioxidant vitamins. Am. J. Pathol. 2007, 170, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.T.; Wei, W.; Ma, B.; Xu, Y.; Liu, W.J.; Wang, Y.; Lv, K.Y.; Tang, H.T.; Wei, D.; Xia, Z.F. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J. Immunol. 2007, 179, 7808–7819. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Shih, R.H.; Lin, C.C.; Chen, J.T.; Yang, C.M. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun. Signal. 2012, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Papaconstantinou, J. p38 Mitogen activated protein kinase (MAPK): A new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert. Opin. Ther. Targets 2016, 20, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Goldman, B.; Radnaa, E.; Kechichian, T.; Menon, R. Silencing P38 MAPK reduces cellular senescence in human fetal chorion trophoblast cells. Am. J. Reprod. Immunol. 2023, 89, e13648. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Richardson, L.; Martin, L.; Jin, J.; Menon, R. Systematic review of p38 mitogen-activated kinase and its functional role in reproductive tissues. Am. J. Reprod. Immunol. 2018, 80, e13047. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Choe, J. Prostaglandin E2 stimulates COX-2 expression via mitogen-activated protein kinase p38 but not ERK in human follicular dendritic cell-like cells. BMC Immunol. 2020, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Parente, R.; Trifirò, E.; Cuozzo, F.; Valia, S.; Cirone, M.; Di Renzo, L. Cyclooxygenase-2 is induced by p38 MAPK and promotes cell survival. Oncol. Rep. 2013, 29, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Mo, L.Q.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef]
- Richardson, L.; Dixon, C.L.; Aguilera-Aguirre, L.; Menon, R. Oxidative stress-induced TGF-beta/TAB1-mediated p38MAPK activation in human amnion epithelial cells. Biol. Reprod. 2018, 99, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, F.; Romero, R.; Chaiworapongsa, T.; Erez, O.; Vaisbuch, E.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; Mazaki-Tovi, S.; Kim, C.J.; et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: A link between the inflammasome and parturition. J. Matern. Fetal Neonatal Med. 2008, 21, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Miller, D.; Leng, Y.; Panaitescu, B.; Silva, P.; Faro, J.; Alhousseini, A.; Gill, N.; et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am. J. Reprod. Immunol. 2018, 79, e12827. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Parvizi, S.T.; Oyarzun, E.; Mazor, M.; Wu, Y.K.; Avila, C.; Athanassiadis, A.P.; Mitchell, M.D. Amniotic fluid interleukin-1 in spontaneous labor at term. J. Reprod. Med. 1990, 35, 235–238. [Google Scholar] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Panaitescu, B.; Miller, D.; Zou, C.; Gudicha, D.W.; Tarca, A.L.; Para, R.; Pacora, P.; Hassan, S.S.; et al. Gasdermin D: In vivo evidence of pyroptosis in spontaneous labor at term. J. Matern. Fetal Neonatal Med. 2021, 34, 569–579. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Motomura, K.; Miller, D.; Garcia-Flores, V.; Galaz, J.; Romero, R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J. Immunol. 2019, 203, 2757–2769. [Google Scholar] [CrossRef]
- Panaitescu, B.; Romero, R.; Gomez-Lopez, N.; Xu, Y.; Leng, Y.; Maymon, E.; Pacora, P.; Erez, O.; Yeo, L.; Hassan, S.S.; et al. In vivo evidence of inflammasome activation during spontaneous labor at term. J. Matern. Fetal Neonatal Med. 2019, 32, 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Stutz, A.; Horvath, G.L.; Monks, B.G.; Latz, E. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol. 2013, 1040, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Panaitescu, B.; Miller, D.; Abrahams, V.M.; Hassan, S.S. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am. J. Reprod. Immunol. 2017, 77, e12648. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Romero, R.; Galaz, J.; Tao, L.; Garcia-Flores, V.; Xu, Y.; Done, B.; Arenas-Hernandez, M.; Miller, D.; Gutierrez-Contreras, P.; et al. Fetal and maternal NLRP3 signaling is required for preterm labor and birth. JCI Insight 2022, 7, e158238. [Google Scholar] [CrossRef] [PubMed]
- Colon-Caraballo, M.; Lee, N.; Nallasamy, S.; Myers, K.; Hudson, D.; Iozzo, R.V.; Mahendroo, M. Novel regulatory roles of small leucine-rich proteoglycans in remodeling of the uterine cervix in pregnancy. Matrix Biol. 2022, 105, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Akgul, Y.; Word, R.A.; Ensign, L.M.; Yamaguchi, Y.; Lydon, J.; Hanes, J.; Mahendroo, M. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J. Clin. Investig. 2014, 124, 5481–5489. [Google Scholar] [CrossRef] [PubMed]
- Pacora, P.; Romero, R.; Maymon, E.; Gervasi, M.T.; Gomez, R.; Edwin, S.S.; Yoon, B.H. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am. J. Obstet. Gynecol. 2000, 183, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.N.; Rao, L.; Sha, Y.; Abdel Fattah, E.; Hyser, J.; Eissa, N.T. p38 MAPK Activity Is Required to Prevent Hyperactivation of NLRP3 Inflammasome. J. Immunol. 2021, 207, 661–670. [Google Scholar] [CrossRef]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Muller, F. The nature and mechanism of superoxide production by the electron transport chain: Its relevance to aging. J. Am. Aging Assoc. 2000, 23, 227–253. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Antimicrobial actions of reactive oxygen species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Oxidative stress: An evolving definition. Fac. Rev. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, M.T.; Burton, G.J.; Jauniaux, E.; Seed, P.T.; Peters, W.H.; Steegers, E.A.; Poston, L. Placental NAD(P)H oxidase mediated superoxide generation in early pregnancy. Placenta 2006, 27, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.H.; Schafer, Z.T. Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid. Redox Signal. 2021, 34, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxid. Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Yung, H.W.; Johns, J.; Spasic-Boskovic, O.; Korolchuk, S.; Jauniaux, E.; Burton, G.J.; Charnock-Jones, D.S. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am. J. Pathol. 2007, 171, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Roychoudhury, S. Reactive Oxygen Species in the Reproductive System: Sources and Physiological Roles. Adv. Exp. Med. Biol. 2022, 1358, 9–40. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Richardson, L.; Sheller-Miller, S.; Zhong, N.; Menon, R. Oxidative stress induces p38MAPK-dependent senescence in the feto-maternal interface cells. Placenta 2018, 67, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Keshari, R.S.; Verma, A.; Barthwal, M.K.; Dikshit, M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 2013, 114, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castro, J.; Florido, J.; Kajarabille, N.; Prados, S.; de Paco, C.; Ocon, O.; Pulido-Moran, M.; Ochoa, J.J. A new approach to oxidative stress and inflammatory signaling during labour in healthy mothers and neonates. Oxid. Med. Cell. Longev. 2015, 2015, 178536. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef]
- Qayyum, N.; Haseeb, M.; Kim, M.S.; Choi, S. Role of Thioredoxin-Interacting Protein in Diseases and Its Therapeutic Outlook. Int. J. Mol. Sci. 2021, 22, 2754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, F.; Yin, Q.; Wang, D.; Han, W.; Zhang, Y. Reactive Oxygen Species Interact with NLRP3 Inflammasomes and Are Involved in the Inflammation of Sepsis: From Mechanism to Treatment of Progression. Front. Physiol. 2020, 11, 571810. [Google Scholar] [CrossRef] [PubMed]
- Dominic, A.; Le, N.T.; Takahashi, M. Loop between NLRP3 Inflammasome and Reactive Oxygen Species. Antioxid. Redox Signal. 2022, 36, 784–796. [Google Scholar] [CrossRef]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojo, C.; Rodríguez-Orozco, A.R. Importance of oxidative damage on the electron transport chain for the rational use of mitochondria-targeted antioxidants. Mini Rev. Med. Chem. 2011, 11, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, S.; Wray, S. Oxytocin: Its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014, 26, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.D.; Panaitescu, B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†. Biol. Reprod. 2019, 100, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome†. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.P.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flis, W.; Socha, M.W. The Role of the NLRP3 Inflammasome in the Molecular and Biochemical Mechanisms of Cervical Ripening: A Comprehensive Review. Cells 2024, 13, 600. https://doi.org/10.3390/cells13070600
Flis W, Socha MW. The Role of the NLRP3 Inflammasome in the Molecular and Biochemical Mechanisms of Cervical Ripening: A Comprehensive Review. Cells. 2024; 13(7):600. https://doi.org/10.3390/cells13070600
Chicago/Turabian StyleFlis, Wojciech, and Maciej W. Socha. 2024. "The Role of the NLRP3 Inflammasome in the Molecular and Biochemical Mechanisms of Cervical Ripening: A Comprehensive Review" Cells 13, no. 7: 600. https://doi.org/10.3390/cells13070600